Electrochemical machining at one point was termed “Action at a distance”. Now there is truly an electrochemical action being designed, where in we can carry out an electrochemical process on a conductive substrate without ever connecting an electrical lead. Yes, recent research and investigations on bipolar electrochemistry makes this possible.
Bipolar behavior had been utilized effectively in electrochemical synthesis, energy generation and storage though conductive substrates action as bipolar plates. In these scenarios, these bipolar plates are strictly current collector and donor and they are not electrochemically active. In the case of bipolar electrochemistry, the conductive substrate that is in the path of current flow between an electrochemical cell conducts electrons through an electrochemical reaction.
So, what is the driving force for current to flow through the conductive plate by carrying out couple of charge transfer process rather than through ionic means? It boils down to the path of least resistance. When the ionic resistance of the electrolyte is much higher than that of the charge transfer resistance, current flow through bipolar electrochemistry is the preferred approach. So for this to happen, electrochemists have to design electrolytes with lower conductivity (quite an unusual exercise!). The beauty here is to carryout reactions on conductive substrates that are electrically floating.
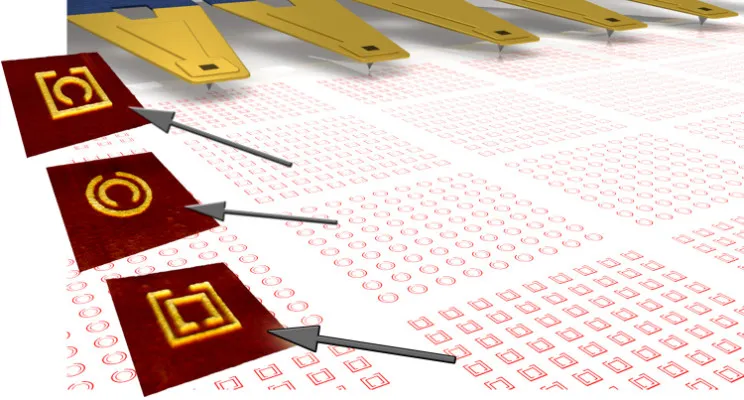
There have been several applications recently using the principle of bipolar electrochemistry. Creation of copper posts on two electrically isolated copper interconnects, creation of conducting particles that are differently decorated, creation of composition graded material on the surface of a conductive substrate etc. Along these lines, T.M. Braun and D. T. Schwartz of University of Washington utilized bipolar electrochemistry to create micro patterning on copper substrate (Journal of The Electrochemical Society, 162 (4) D180-D185 (2015)).
They combined their Electrochemical Printing (EcP) technique with bipolar electrochemistry and created a Scanning Bipolar Cell (SBC). Using SBC, they demonstrated creation of microstructures on surface of copper substrate. They also developed dimensionless bipolar current efficiency, with Wagner number derived from their experimental system. This will help in quick experimental evaluations of the scaled down version of their approach.
To learn more, you can access the full article which is available via open access at 10.1149/2.1031504jes