Photocatalysis can be defined as the change in the rate of chemical transformation in the presence of light, assisted by a catalyst that is capable of absorbing light and turn it into a useful energy source. Heterogeneous photocatalysis employ semiconductor materials (mainly transition metal oxides), this technology has found important applications such as water splitting for energy generation or pollutant degradation.
For real-life applications, photoactive materials are required to have strong oxidation power, high photoactive efficiency, stability, and a reasonable cost. TiO2 is a metal oxide that is widely used for photocatalysis, however, due to its large bandgap and rapid recombination of electron-hole pairs, it requires modifications in order to increase its efficiency.
A series of modifications can be used to tune up the photoactive material properties, which include metal and non-metal doping, structural modification, dye sensitization, combination with other semiconductors, and immobilization on support structures. All these modifications aim to reduce the bandgap of the material allowing to increase the absorption of light in the visible spectrum, therefore increasing their efficiency in light photon utilization.
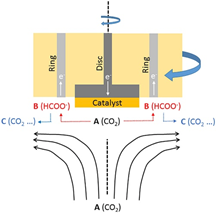
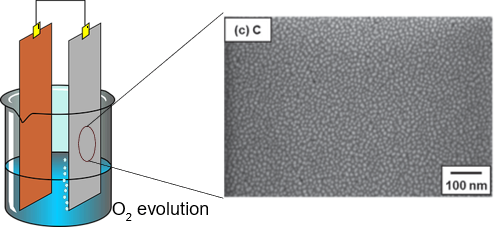
During the fabrication step of a photocatalyst, several factors are affecting the final material properties. For example, anodization is a technique commonly used for the generation of metal oxide layers on the surface of metal materials. Anodization can lead to different results depending on factors like raw material, electrolyte, and oxygen concentration. In a recent study, Ni et al at the College of Environment and Resources of the Southwest University suggested a novel approach for the formation of TiO2 from the anodization of TiB2(Ni et al., 2020). They found that the anodization was dependent on the crystal orientation of the pressed and sintered TiB2: a monolith with exposed (100) surface was able to form a TiO2 layer while the one with exposed (001) produced a gel in the solution and after hydrothermal treatment, it can be used to produce B-doped nanocrystals. This approach allowed the formation of B-doped TiO2 nanocrystals without further preparation steps or the addition of chemicals.
In the study performed by Ni et al, the TiB2 monolith led to the fabrication of a photoelectrochemical electrode for water splitting. The electrode showed a photocurrent 10 times higher than that generated from Ti0. On the other hand, B-doped anatase nanocrystals can be implemented for dye removal in wastewaters. The electrode was tested for the degradation of Rhodamine B, showing a degradation efficiency 4 times higher than undoped TiO2.
A systematic approach needs to be taken to optimize the fabrication of photoactive materials; this will allow an understanding of the mechanisms involved during the formation of the layers. In this way, the technique used will be able to control critical parameters at the anodization step to enhance the efficiency of the photocatalytic materials for several purposes.
Watch the recording of the discussion in our Weekly Science Review:
Full article is available via open access at https://iopscience.iop.org/article/10.1149/1945-7111/abad67
Ni, C., Tang, Y., Abdellatif, H. R. S., Huang, X., Xie, D., & Ni, J. (2020). Boron-Doped TiO 2 from Anodization of TiB 2 for Efficient Photocatalysis . Journal of The Electrochemical Society, 167(12), 126505. https://doi.org/10.1149/1945-7111/abad67