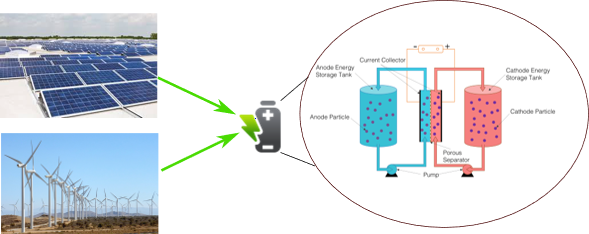
The growing demand for renewable energy has created new challenges, this owing to the intermittent nature of solar and wind energy sources. Several energy storage systems have been proposed as a viable solution for coupling renewable systems in remote locations. Among them, Redox Flow Batteries (RFBs) is considered one of the most promising technologies offering advantages such as Low Levelized Cost of Storage (LCOS), easy scalability, long life cycles (20000-25000), enhanced safety, and zero emissions.
Redox Flow Batteries are electrochemical devices that store energy using chemicals as energy carriers which are usually found in liquid form. The cell is separated by a selective membrane (usually a proton exchange membrane) to avoid loss of capacity due to cross-mixing. The liquids containing the redox-active species are pumped to the cell during charge/discharge cycles. In the scientific literature, we can find a wide variety of chemistries and configurations for RFBs. However, iron-based flow batteries are commonly used due to the low cost of active redox materials, availability, and environmental friendliness.
The energy storage capacity and energy density of RFBs rely on several factors, for instance, the cell voltage is dependent on the redox potential of the half-cell reactions. Typical cell voltages range from 1 to 2.4 V. The storage capacity is dependent on the concentration of the redox species, higher concentrations mean higher capacity. Despite the advantages of iron-based RFBs, some challenges remain regarding long-term chemical stability and durability. Since the iron species are dissolved in aqueous solutions, parasitic reactions such as hydrogen evolution can limit the performance and life of the battery.
Low-cost iron sources can be found in the industry, for example, steel mills generate enormous amounts of iron sulfate as waste. Taking advantage of such materials can be beneficial for energy storage systems. In a recent study, Yang et al at the University of Southern California proposed an innovative RFB based on iron sulfate and an organic molecule (anthraquinone disulfonic acid-ADQS) having a relatively low cell voltage of 0.62 V (Yang et al., 2020).
Another major challenge for RFBs is the species cross-over through the proton exchange membrane (PEM), which is considered one of the related issues to capacity fade. Most RFBs are operated in an asymmetrical mode, where one species is used for anodic reactions while another is used for the cathode. The exploration of other operation modes can help to understand capacity loss and improve durability. In the work presented by Yang et al, the authors proposed the operation in a symmetrical mode, where both electrolytes were mixed in separated tanks for the positive and negative electrolytes. According to the authors, despite that the species are mixed in the solution, selective reactions are taking place at each electrode. The results proved that in such a mode, over 500 cycles can be achieved with a negligible capacity fade of 7.6 × 10-5 % per cycle with sustained coulombic efficiency of 99%. Despite the lower cell voltage in comparison with other chemistries, this alternative can help to meet the requirements of cost, durability, and scalability for large scale applications.
Watch our discussion of this paper in our Weekly Science Review:
For more details, please refer to the full article available at: https://iopscience.iop.org/article/10.1149/1945-7111/ab84f8
Yang, B., Murali, A., Nirmalchandar, A., Jayathilake, B., Prakash, G. K. S., & Narayanan, S. R. (2020). A Durable, Inexpensive and Scalable Redox Flow Battery Based on Iron Sulfate and Anthraquinone Disulfonic Acid. Journal of The Electrochemical Society, 167(6), 060520. https://doi.org/10.1149/1945-7111/ab84f8