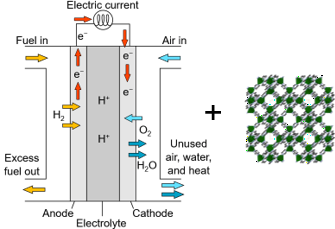
To this date Pt and Pt-based materials are known as the best catalysts for ORR, however, these materials often suffer from easy poisoning and are very expensive. Therefore, it is of great interest to develop new electrocatalysts with high efficiency and low cost. In the search for a novel catalyst, the production of highly efficient electrocatalyst based on rich earth elements has become a common practice.
Novel materials such as Metal-Organic Frameworks (MOFs) have been raised as a promising element for the fabrication of catalyst. MOFs are highly ordered structures consisting of a metal center bridged together by organic ligands. These novel materials can be used as precursors for the fabrication of carbon-based materials. Their attractive properties include a super-high surface area, high thermal and chemical stability, high reactivity, and relatively easy fabrication.
To better understand the process of ORR, it is important to explore the effect of active components such as transition metals. Based on theoretical estimations, Cu-based catalysts can have higher activity than others like Fe, Co, Ni, etc. The major drawback of Cu-based materials is their reduced chemical and thermal stability, and poor conductivity. Recent research has been done for the generation of Cu, carbon, or nitrogen nanostructures which can be used for the fabrication of catalysts for ORR.
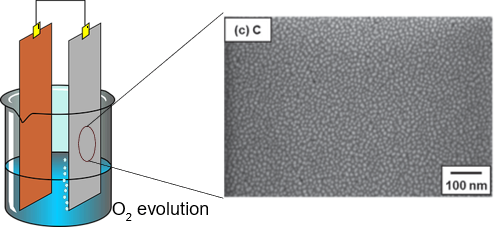
An example of the combination of these materials and techniques can be found in the study presented by Parkash at Shaanxi Normal University. He proposed the fabrication of Cu promoted nitrogen-doped carbon based on Cu/ZIF-8 MOF (Parkash, 2020). The synthesis of the novel catalysts has as final step pyrolysis under an inert atmosphere of nitrogen, this step proved to be determinant on the electrocatalytic activity of the material. The pyrolysis step was carried out using temperatures of 500, 600, 700, 800, and 900 °C. The results showed that the catalyst fabricated at 800 °C has an outstanding electrocatalytic activity in basic media and exhibited higher stability compared to the Pt/C catalyst. According to the author, the impressive catalytic bifunctional performance can be attributed to the strong synergy between Cu (II)-N ligands and Cu0 nanoparticles, rich active centers, and rapid mass transfer. Also, after the accelerated aging test, the catalyst retained its activity even after 1000 cycles, this demonstrates the high stability achieved through the fabrication process based on the ZIF-8 MOF.
Similar studies need to be conducted to understand the mechanism associated with ORR. Metal-N co-doping strategy will significantly improve the catalyst’s electrocatalytic operation, which provides a valuable guideline for the design of a new non-noble N-doped carbon-based ORR catalyst. This work will promote the development of effective, low cost, nanostructured non-noble metal electrocatalysts for renewable energy conversion and storage.
Watch the discussion of this paper in our weekly science review below.
For more details please refer to the full paper available at https://iopscience.iop.org/article/10.1149/1945-7111/abaaa5
Parkash, A. (2020). Copper Doped Zeolitic Imidazole Frameworks (ZIF-8): A New Generation of Single-Atom Catalyst for Oxygen Reduction Reaction in Alkaline Media. Journal of The Electrochemical Society, 167(15), 155504. https://doi.org/10.1149/1945-7111/abaaa5