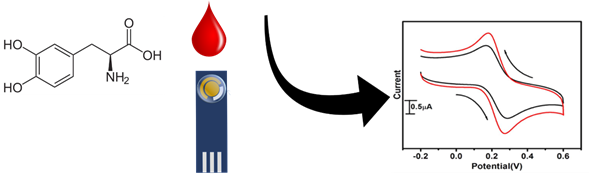
Serotonin (5-hydroxytryptamine) is a neurotransmitter that plays a key role in several physiological processes related to sleep, mood regulation, appetite, and cardiovascular function. Low levels of serotonin are related to migraine, anxiety, depression, eating disorder, and blood clotting sudden infant death syndrome. While high levels of serotonin can cause potentially fatal effects and toxicity. Owing to the importance of this molecule, it is crucial to determine its physiological levels for an opportunist diagnosis. The standard human level is in the range of 0.25-0.74 mM according to the US Department of Health, Human Services, and National Institutes of Health.
The determination of serotonin can be carried out in urine and serum, and several analytical methods are currently employed including High-Performance Liquid Chromatography (HPLC), fluorimetry, mass spectrometry, enzyme-linked immune sorbent assay (ELISA), and electrochemical methods. Among these methods, an electrochemical determination is considered the best option using simple procedures like cyclic voltammetry and differential pulse voltammetry. For this matter, electroanalytical techniques have proved to be an effective, painless, and low-cost method for quick diagnosis.
The implementation of nanomaterials has attracted attention in the field of sensors, allowing to increase the sensitivity and stability of sensor devices. Electrochemical sensors require stable materials in different conditions of pH and temperature. Typical construction of the working electrode consists of the functionalization of a carbon-based material with nanomaterials containing metals, metal oxides, metal sulfide, etc. Among metal oxides, ZrO2 (zirconia) is a very prominent material possessing high stability and hardness at high temperatures.
In a recent report, Bullapura et al explored the use of ZnO2 nanoparticles (ZnO2-NPs) for the electrochemical detection of serotonin (Bullapura Matt et al., 2020). In this work, an easy method for the fabrication of the nanoparticles consisted of a gel-combustion method. The formation of nanoparticles was confirmed by SEM and TEM analysis, ZnO2-NPs showed an average particle size of 40 nm. After the synthesis, the zirconium nanoparticles were added to a carbon paste electrode.
During the electrochemical characterization, the carbon paste electrode with the ZnO2-NPs showed improved catalysis towards serotonin in comparison to the bare carbon material. To identify the effect of pH on the catalytic activity, the authors performed the determination at different pH values in the range of 6.2-7.4. The maximum electrocatalytic activity was found at 7.4 which is also physiological pH, this fact indicates that serotonin determination can be carried out without sample pretreatment. The interferences were evaluated versus dopamine, in this case, the differential pulse voltammetry technique help to separate the contribution of each molecule and no interference was found. The method proposed by Bullapura et al showed to be highly sensitive allowing the determination of serotonin in concentrations of 10-50 µM with a limit of detection of 0.585 µM.
The implementation of novel materials at the nanoscale can help to increase the sensitivity, selectivity, and stability of electrochemical sensors for the detection of different molecules of interest for medical applications.
Watch the discussion of this paper in our weekly science review:
For more details of this study, please refer to the full article available at https://iopscience.iop.org/article/10.1149/1945-7111/abb835
Bullapura Matt, S., Shivanna, M., Manjunath, S., Siddalinganahalli, M., & Siddalingappa, D. M. (2020). Electrochemical Detection of Serotonin Using t-ZrO 2 Nanoparticles Modified Carbon Paste Electrode. Journal of The Electrochemical Society, 167(15), 155512. https://doi.org/10.1149/1945-7111/abb835