Electrolytic processes at the industrial scale are widely used for different purposes. The most common processes involve oxygen and chlorine production in anodic reactions. In such electrolytic processes, the electrode material is crucial for long-term operation and efficiency. Among the electrode materials, titanium-coated electrodes are used as insoluble anodes. In these electrodes, the active electrocatalytic materials are usually mixed-oxides composed of platinum group metal oxides such as IrO2 and RuO2. For instance, IrO2-Ta2O5/Ti electrodes are used in the electrogalvanizing and electrotinning of steel processes due to their high durability in acidic aqueous solutions.
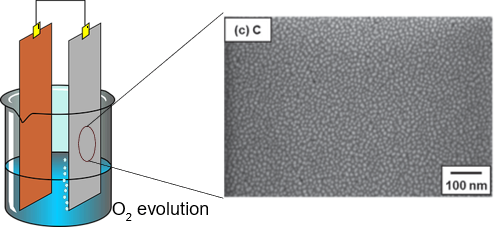
Despite the high chemical stability of the Ti-coated electrodes, some challenges remain in terms of reaction selectivity. Secondary reactions not only can cause a decrease in the process efficiency but can lead to the deposition of byproducts on the electrode surface, affecting the electrode lifetime. For example, in the electrolytic copper foil production, IrO2-Ta2O5/Ti anodes are used for oxygen evolution in acidic electrolyte solutions. Although copper sulfate electrolyte is used for this process, Pb (II) ions can be found as an impurity in the solution. During operation lead oxidation is a side reaction leading to PbO2 electrodeposition on the electrode surface. The deposited PbO2 can be further reduced to a non-conductive PbSO4 layer, increasing cell voltage over time.
Typical fabrication of IrO2-Ta2O5/Ti electrodes includes thermal deposition at high temperatures about 450-500 °C. Material characterization techniques revealed that the catalytic layer is composed of crystalline IrO2 and amorphous TaO2. Also, segregated IrO2-Ta2O5 particles were found on the surfaces along with flat areas and cracks. Therefore, it is very important to elucidate the key parameters to obtain a more homogeneous catalyst since it has been proved that surface morphology has a direct impact on electrocatalytic activity.
Recent efforts in the study of IrO2-Ta2O5/Ti electrodes were presented by Kawaguchi and Morimitsu, they investigated the effect of the thermal decomposition temperature on both the surface morphology and the electrocatalytic activity of the resulting electrodes (Kawaguchi & Morimitsu, 2020). The experiments were conducted at different temperatures (470 and 380 °C) and IrO2/Ta2O5 ratios. The results showed that at low temperature a nano/amorphous hybrid structure of IrO2 particles was created. The nanoparticles were highly dispersed in an amorphous Ta2O5 matrix. Regarding the electrocatalytic activity, the oxygen evolution was accelerated with an increasing Ir ratio at the low temperature. Moreover, anodic PbO2 deposition was completely suppressed even in the presence of an electrolytic solution containing 100 ppm PbSO4 in acidic conditions. On the other hand, the PbSO4 deposition on electrodes fabricated at 470 °C was confirmed.
Studies like the one of Kawaguchi and Morimitsu can help to improve the overall performance of Ti-coated electrodes at the industrial scale. However, these electrodes need further improvement regarding durability. Nevertheless, the study sets a precedent for future investigations.
Watch the discussion of this paper in our weekly science review:
For more details on this study, please refer to the full articles available at https://iopscience.iop.org/article/10.1149/1945-7111/abb7e8.
Kawaguchi, K., & Morimitsu, M. (2020). Reaction Selectivity of IrO 2 -Based Nano/Amorphous Hybrid Oxide-Coated Titanium Anodes in Acidic Aqueous Solutions: Oxygen Evolution and Lead Oxide Deposition. Journal of The Electrochemical Society, 167(13), 133503. https://doi.org/10.1149/1945-7111/abb7e8